Gold is a dense, soft, shiny, malleable and ductile metal. It is a chemical element with the symbol Au (aurum in Latin, meaning glow of sunrise) and atomic number 79.
It has a bright yellow color and luster traditionally considered attractive, which it maintainswithout oxidizing in air or water. Chemically, gold is a transition metal and a group 11 element. It is one of the least reactive chemical elements solid under standard conditions. The metal therefore occurs often in free elemental (native) form, as nuggets or grains in rocks, in veins and in alluvial deposits. Less commonly, it occurs in minerals as gold compounds, usually with tellurium.
Gold resists attacks by individual acids, but it can be dissolved by the aqua regia (nitro-hydrochloric acid), so named because it dissolves gold. Gold also dissolves in alkaline solutions of cyanide, which have been used in mining. It dissolves in mercury, forming amalgam alloys; is insoluble in nitric acid, which dissolves silver and base metals, a property that has long been used to confirm the presence of gold in items, giving rise to the term the acid test.
This metal has been a valuable and highly sought-after precious metal for coinage, jewelry, and other arts since long before the beginning of recorded history. Gold standards have sometimes been a monetary policies, but were widely supplanted by fiat currency starting in the 1930s. The last gold certificate and gold coin currencies were issued in the U.S. in 1932. In Europe, most countries left the gold standard with the start of World War I in 1914 and, with huge war debts, failed to return to gold as a medium of exchange.
A total of 171,300 tonnes of gold have been mined in human history, according to GFMS as of 2011. This is roughly equivalent to 5.5 billion troy ounces or, in terms of volume, about 8876 m 3 , or a cube 20.7 m on a side. The world consumption of new gold produced is about 50% in jewelry, 40% in investments, and 10% in industry.
Besides its widespread monetary and symbolic functions, gold has many practical uses in dentistry, electronics, and other fields. Its high malleability, ductility, resistance to corrosion and most other chemical reactions, and conductivity of electricity led to many uses of gold, including electric wiring, colored-glass production and gold leafing.
Most of the Earth&’s gold lies at its core, the metal's high density having made it sink there in the planet’s youth. Virtually all of the gold that mankind has discovered is considered to have been deposited later by meteorites which contained the element, with the asteroid that formed Vredefort crater being implicated in the formation of the largest gold mining region on earth – Witwatersrand basin.
Etymology
“Gold” is cognate with similar words in many Germanic languages, deriving via Proto-Germanic *gulþą from Proto-Indo-European *gʰel- (“yellow/green”).
The symbol Au is from the Latin: aurum, according to some sources meaning "shining dawn” from Sabine ausum "glowing dawn”although according to definitions within Latin dictionaries the meaning of the word aurum extends only to the same as today’s reference to the metal. The disagreement between definitions is possibly due to the accumulation of evidence from archaeology of the original anciency of the metal in civilization; in reference to "the dawn of civilization", and in this respect has become the adopted modern meaning, disassociated from the original etymological Latin.
Characteristics
Gold is the most malleable of all metals; a single gram can be beaten into a sheet of 1 squaremeter, or an ounce into 300 square feet. Gold leaf can be beaten thin enough to become transparent. The transmitted light appears greenish blue, because gold strongly reflects yellow and red. Such semi-transparent sheets also strongly reflect infrared light, making them useful as infrared (radiant heat) shields in visors of heat-resistant suits, and in sun-visors for spacesuits.
Gold readily creates alloys with many other metals. These alloys can be produced to modify the hardness and other metallurgical properties, to control melting point or to create exotic colors (see below). Gold is a good conductor of heat and electricity and reflects infrared radiation strongly. Chemically, it is unaffected by air, moisture and most corrosive reagents, and is therefore well suited for use in coins and jewelry and as a protective coating on other, more reactive, metals. However, it is not chemically inert. Gold is almost insoluble, but can be dissolved in aqua regia.
Common oxidation states of gold include +1 (gold (I) or aurous compounds) and +3 (gold (III) or auric compounds). Gold ions in solution are readily reduced and precipitated out as gold metal by adding any other metal as the reducing agent. The added metal is oxidized and dissolves allowing the gold to be displaced from solution and be recovered as a solid precipitate.
High quality pure metallic gold is tasteless and scentless; in keeping with its resistance tocorrosion (it is metal ions which confer taste to metals).
In addition, gold is very dense, a cubic meter weighing 19,300 kg. By comparison, the density of lead is 11,340 kg/m 3 , and that of the densest element, osmium, is 22,610 kg/m 3 .
Color :
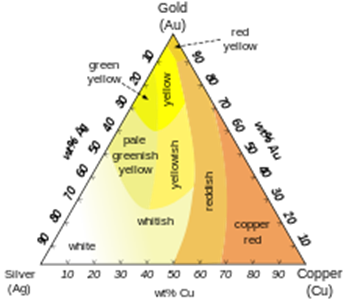
Different colors of Ag-Au-Cu alloys
Whereas most other pure metals are gray or silvery white, gold is yellow. This color is determined by the density of loosely bound (valence) electrons; those electrons oscillate as a collective “plasma” medium described in terms of a quasiparticle called plasmon. The frequency of these oscillations lies in the ultraviolet range for most metals, but it falls into the visible range for gold due to subtle relativistic effects that affect the orbitals around gold atoms. Similar effects impart a golden hue to metallic caesium (see relativistic quantum chemistry).
Common colored gold alloys such as rose gold can be created by the addition of various amounts of copper and silver, as indicated in the triangular diagram to the left. Alloys containing palladium or nickel are also important in commercial jewelry as these produce white gold alloys. Less commonly, addition of manganese, aluminium, iron, indium and other elements can produce more unusual colors of gold for various applications.
Isotopes:
Gold has only one stable isotope, 197 Au, which is also its only naturally occurring isotope. Thirty-six radioisotopes have been synthesized ranging in atomic mass from 169 to 205. The most stable of these is 195 Au with a half-life of 186.1 days. The least stable is 171 Au, which decays by proton emission with a half-life of 30 µs. Most of gold’s radioisotopes with atomic masses below 197 decay by some combination of proton emission, α decay, and β+ decay. The exceptions are 195 Au, which decays by electron capture, and 196 Au, which decays most often by electron capture (93%) with a minor β- decay path (7%). All of gold’s radioisotopes with atomic masses above 197 decay by β- decay.
At least 32 nuclear isomers have also been characterized, ranging in atomic mass from 170 to 200. Within that range, only 178 Au, 180 Au, 181 Au, 182 Au, and 188 Au do not have isomers. Gold’s most stable isomer is 198m2 Au with a half-life of 2.27 days. Gold’s least stable isomer is 177 m2 Au with a half-life of only 7 ns. 184 m1 Au has three decay paths: β+ decay, isomeric transition, and alpha decay. No other isomer or isotope of gold has three decay paths.


